Researchers examined midostaurin resistance or sensitivity in a cohort of patients with acute myeloid leukemia.
The Trending with Impact series highlights Oncotarget publications attracting higher visibility among readers around the world online, in the news, and on social media—beyond normal readership levels. Look for future science news about the latest trending publications here, and at Oncotarget.com.
—
Acute myeloid leukemia (AML) is a heterogeneous malignancy that most commonly affects older adults, 60 years of age and older. NPM1, DNMT3A, and FLT3 are the most common genomic alterations found within this disease. In about 30% of AML patients, FLT3 is mutated. Midostaurin was the first FDA approved FLT3 inhibitor for AML. While Midostaurin has a successful overall survival benefit, both primary and secondary resistance remains common.
“A subtype of AML, classified by the presence of a FLT3-Internal Tandem Duplication (ITD) mutation, tends to have a worse prognosis with early relapse and death [5].”
Researchers from Oregon Health and Science University and Howard Hughes Medical Institute conducted a study to identify features that may predict response to midostaurin in FLT3 mutant and wild-type samples. They performed an ex vivo drug sensitivity screen on primary and relapsed AML samples, with corresponding targeted sequencing and RNA sequencing. The paper was entitled: “Genomic markers of midostaurin drug sensitivity in FLT3 mutated and FLT3 wild-type acute myeloid leukemia patients.”
The Study
In order to understand the impact that different genomic alterations have on midostaurin response, 214 patients were functionally assessed with midostaurin and their FLT3 status was annotated. Of these patients, the researcher identified 193 primary and 21 relapse AML samples from the Beat AML publicly available dataset. Risk groups within the cohort were as follows: 73 samples were favorable risk, 59 samples were intermediate, and 68 were adverse. The median age of patients in the cohort was 61, with 52% male and 48% female.
“We hypothesized that there are additional genomic alterations and gene expression changes outside of FLT3-ITD mutations that can influence AML sample resistance or sensitivity to midostaurin and aimed to further characterize these factors.”
Drug sensitivity screening, RNA sequencing/expression analysis, custom gene panel (GeneTrails) sequencing and variant detection, exome sequencing and variant detection, internal FLT3-ITD and NPM1 mutation detection, derivation of FLT3-ITD and NPM1 consensus calls, ex vivo functional drug screens, and statistical analysis were the methods used to observe the impact of genomic alterations on midostaurin response.
“Our research explored the multi-targeted nature of midostaurin and suggested a number of molecular mutational patterns that correlated with midostaurin drug sensitivity and resistance in both FLT3-ITD mutated and FLT3-ITD wild-type AML patient samples.”
Results
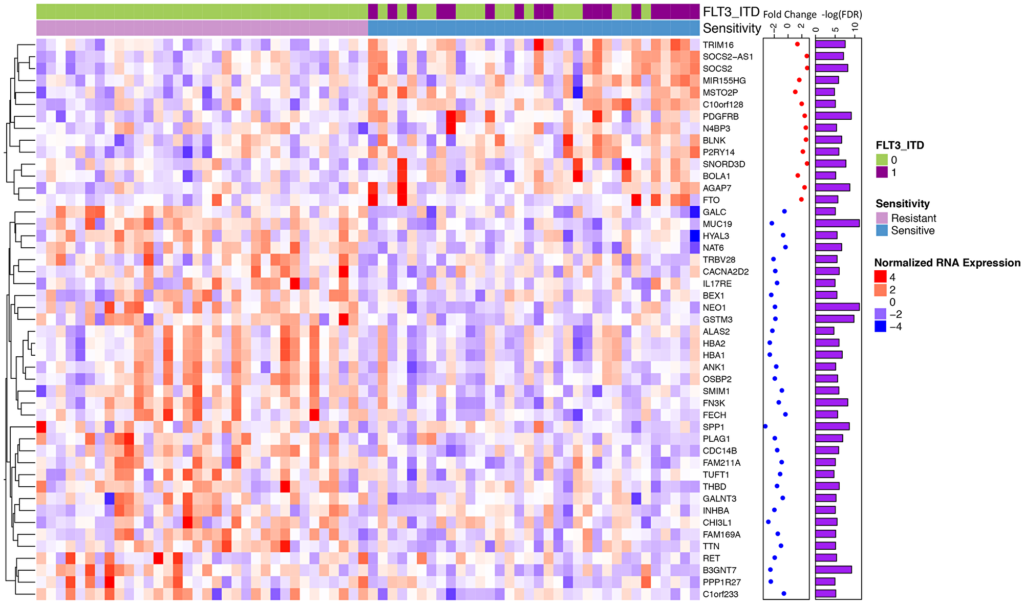
The researchers observed specific point mutations and gene expression patterns that they believe explain why there is a range of responses to midostaurin treatment. In the FLT3-ITD positive cohort, increased expression of the oncogene RGL4 (and regulator of the Ras-Raf-MEK-ERK cascade) correlated with poorer midostaurin response. In the FLT3-ITD negative cohort, KRAS mutations correlated with a poorer midostaurin response.
“We also observed that 16 / 34 of the most sensitive samples did not harbor a FLT3 mutation and a majority of differentially expressed genes were independent of FLT3 status.”
Conclusion
The authors point out that additional research studies will be needed given that their sample cohort was relatively small. They also note that since there are multiple FLT3 inhibitors available, it is important to understand the sensitivity mechanisms of each intervention in order to better personalize therapy for chemo-refractory or relapsed AML patients.
“Overall, we identify genomic alterations that correlate with midostaurin response independent of FLT3-ITD status, propose that Ras-Raf-MEK-ERK inhibition in combination therapy could limit resistance to midostaurin, and suggest that within the overall AML population there may be therapeutic benefit of midostaurin in patients with certain expression profiles.”
Click here to read the full scientific study, published in Oncotarget.
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read—without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.