In this new study, researchers investigated a promising new approach to acute myeloid leukemia (AML) therapy by combining multiple drugs to enhance cytotoxic effects on AML cells.
—
Acute myeloid leukemia (AML) is a cancer characterized by the rapid growth of abnormal white blood cells that accumulate in the bone marrow and interfere with the production of normal blood cells. ABT199, also known as venetoclax, is a targeted therapy that inhibits the BCL-2 protein, which is often overexpressed in AML cells and contributes to their survival. By blocking this protein, venetoclax can trigger apoptosis, or programmed cell death, in cancer cells. Thiotepa, a DNA alkylating agent, has been used in conditioning regimens for hematopoietic stem cell transplantation (HSCT) but its combination with ABT199/venetoclax has not been thoroughly explored, until now.
In a new study, researchers Benigno C. Valdez, Bin Yuan, David Murray, Jeremy L. Ramdial, Uday Popat, Yago Nieto, and Borje S. Andersson from The University of Texas MD Anderson Cancer Center and the University of Alberta investigated a promising new approach to AML therapy by combining multiple drugs to enhance cytotoxic effects on AML cells. On March 14, 2024, their new research paper was published in Oncotarget’s Volume 15, entitled, “ABT199/venetoclax synergism with thiotepa enhances the cytotoxicity of fludarabine, cladribine and busulfan in AML cells.”
“The results may provide relevant information for the design of clinical trials using these drugs to circumvent recognized drug-resistance mechanisms when used as part of pre-transplant conditioning regimens for AML patients undergoing allogenic HSCT.”
The Study
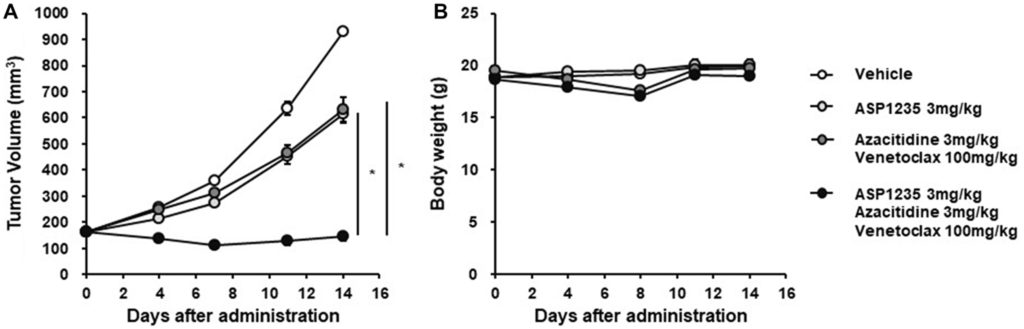
In this study, the researchers demonstrated a notable synergistic effect between ABT199/venetoclax and thiotepa, significantly amplifying cytotoxicity against AML cells. This effect was further magnified when these drugs were combined with fludarabine, cladribine, and busulfan, well-established chemotherapeutic agents renowned for their efficacy in AML treatment.
One pivotal discovery of the research lies in elucidating the molecular mechanism behind this heightened cytotoxicity. The combined drug regimen led to increased cleavage of Caspase 3, PARP1, and HSP90, recognized markers of apoptosis, indicative of a robust activation of the cell death pathway. Additionally, heightened Annexin V positivity, an indicator of early apoptosis stages, was observed, suggesting the effective initiation of cell death in AML cells.
The investigation also shed light on an augmented DNA damage response, evidenced by elevated levels of γ-H2AX, P-CHK1 (S317), P-CHK2 (S19), and P-SMC1 (S957). These markers imply that the drug combination not only induces apoptosis but also contributes to the accumulation of DNA damage in AML cells, further fostering their demise.
Another significant outcome was the activation of stress signaling pathways, reflected in increased levels of P-SAPK/JNK (T183/Y185) and decreased P-PI3Kp85 (Y458). These alterations indicate cellular stress induced by drug treatment, potentially heightening sensitivity to the cytotoxic effects of the combination therapy.
Furthermore, the study addressed the pressing issue of drug resistance, commonly encountered in AML treatment. The five-drug combination notably decreased the levels of BCL-2, BCL-xL, and MCL-1, proteins associated with resistance to venetoclax, suggesting potential efficacy in overcoming resistance and improving treatment outcomes for AML patients. Various AML cell lines, including those with P53-negative and FLT3-ITD-positive mutations associated with poor prognosis, were subjected to the drug combination.
Results & Conclusion
The results exhibited promising activity of the combination therapy against these challenging cell lines. Moreover, extending the findings to clinical relevance, the drug combination was tested on leukemia patient-derived cell samples, revealing enhanced activation of apoptosis, which hints at potential effectiveness in a clinical setting and provides a basis for future clinical trials.
The implications of this research are profound, offering a novel strategy for conditioning regimens in AML patients undergoing HSCT. Combining ABT199/venetoclax and thiotepa with fludarabine, cladribine, and busulfan presents a promising approach for eradicating AML cells and preparing patients for stem cell transplantation. In conclusion, the study signifies a significant advancement in combating AML. The synergistic effects observed in combining ABT199/venetoclax with thiotepa and other chemotherapeutic agents pave the way for enhancing treatment regimens. This research sets the stage for future clinical trials and the potential development of more effective therapies for AML patients.
“The results provide a rationale for clinical trials using these two- and five-drug combinations as part of a conditioning regimen for AML patients undergoing HSCT.”
Click here to read the full research paper in Oncotarget.
—
Oncotarget is an open-access, peer-reviewed journal that has published primarily oncology-focused research papers since 2010. These papers are available to readers (at no cost and free of subscription barriers) in a continuous publishing format at Oncotarget.com.
Oncotarget is indexed and archived by PubMed/Medline, PubMed Central, Scopus, EMBASE, META (Chan Zuckerberg Initiative) (2018-2022), and Dimensions (Digital Science).
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact media@impactjournals.com.