In this editorial, researchers from Japan discuss their recent study investigating the role of p53 in preventing the development of extrahepatic biliary cancer.
—
Extrahepatic biliary cancer is a specific type of biliary cancer that occurs outside the liver. It is considered rare, quite serious and often symptomless until later stages. The average age at diagnosis is 72. Extrahepatic biliary cancer typically involves the bile ducts, which carry bile from the liver and gallbladder to the small intestine. It can also involve the gallbladder, which plays a role in the digestion of fats by storing, concentrating and releasing bile as needed.
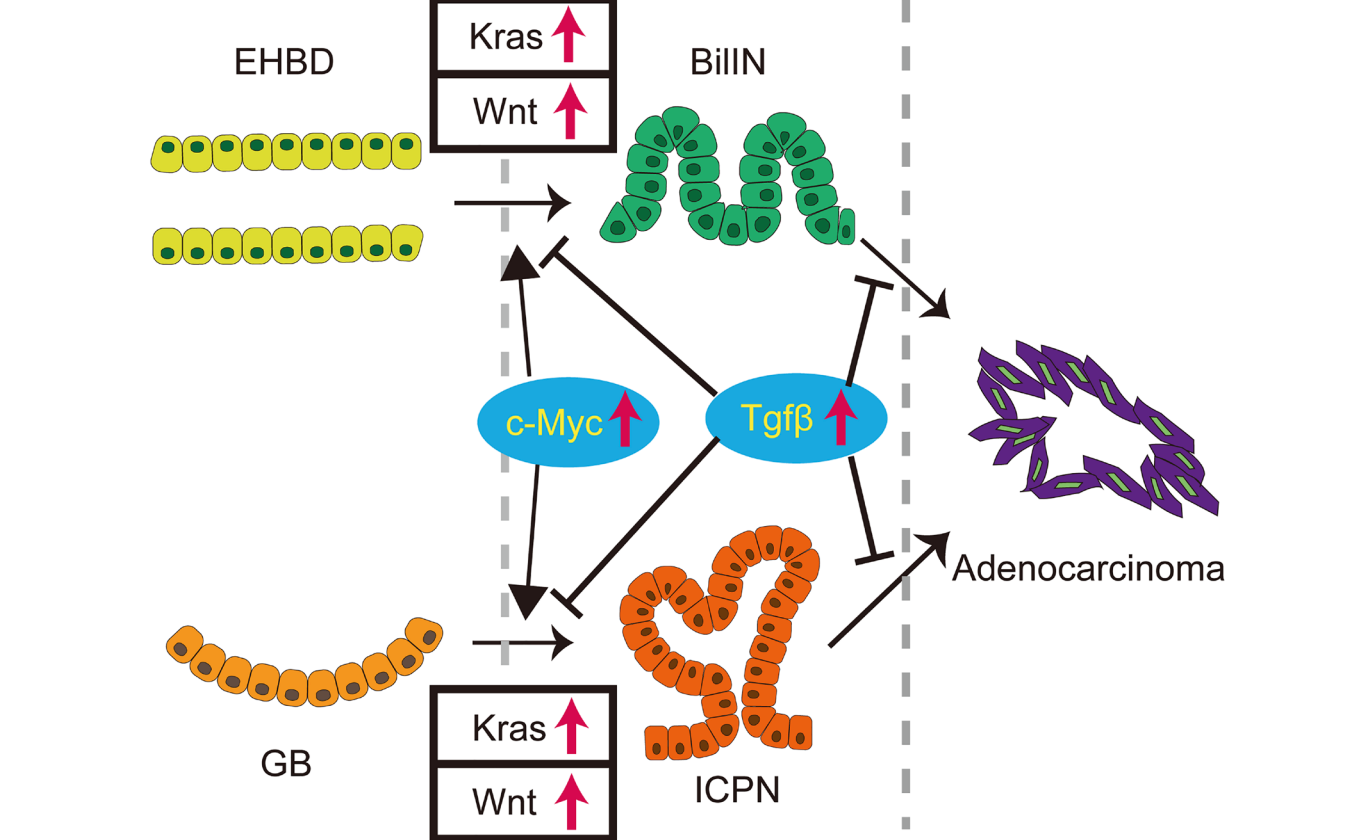
One of the main genetic factors that contribute to biliary cancer is the mutation of Kras, a gene that regulates cell growth and division. Mutated Kras can cause cells to grow uncontrollably and form tumors. Another important genetic factor is the mutation of p53, a gene that normally acts as a guardian of the genome and triggers cell death or repair when DNA damage occurs. Mutated p53 can impair this function and allow cells to survive and accumulate more mutations.
“[…] the exact role of p53 in the development of extrahepatic biliary cancer remains elusive.”
In a new editorial paper, researchers Munemasa Nagao, Kenta Mizukoshi, Shinnosuke Nakayama, Mio Namikawa, Yukiko Hiramatsu, Takahisa Maruno, Yuki Nakanishi, Tatsuaki Tsuruyama, Akihisa Fukuda, and Hiroshi Seno from Kyoto University Graduate School of Medicine discussed their recent study exploring the role of p53 in preventing the development of extrahepatic biliary cancer. On March 31, 2023, their editorial was published in Oncotarget, entitled, “p53 protects against formation of extrahepatic biliary precancerous lesions in the context of oncogenic Kras.”
The Editorial
The authors of the editorial discuss their 2022 study using a mouse model to investigate how Kras and p53 mutations interact in the development of extrahepatic biliary cancer. They found that mice with Kras activation and p53 inactivation developed lesions resembling human biliary neoplasms in the bile duct and gallbladder. These lesions are considered to be precursors of invasive biliary cancer.
“In this study, we found that simultaneous activation of Kras and inactivation of p53 induces biliary neoplasms that resemble human biliary intraepithelial neoplasia in the extrahepatic bile duct and intracholecystic papillary tubular neoplasm in the gall bladder in mice.”
However, they also found that p53 inactivation was not enough for the progression of these lesions into invasive cancer in the presence of oncogenic Kras within the observation period. This was also true when they added another genetic alteration, namely the activation of the Wnt signaling pathway, which is known to promote tumorigenesis in various cancers.
Therefore, they concluded that p53 has a protective role against the formation of extrahepatic biliary precancerous lesions in the context of oncogenic Kras. They suggested that additional genetic or environmental factors may be required for the malignant transformation of these lesions into invasive cancer.
Conclusions
The study the authors described in their recent editorial paper provides new insights into the molecular mechanisms underlying extrahepatic biliary cancer and highlights the importance of p53 as a barrier against tumorigenesis. It also raises questions about what other factors may contribute to biliary cancer progression and how they can be targeted for prevention or treatment. This work received support from a number of institutions, including Grants-in-Aid KAKENHI, the Japan Agency for Medical Research and Development, the Princess Takamatsu Cancer Research Fund, the Mochida Foundation, the Mitsubishi Foundation, the Uehara Foundation, the Naito Foundation, the Kobayashi Foundation, the Simizu Foundation, the Japan Foundation for Applied Enzymology, the SGH Foundation, the Kanae Foundation, Bristol Myers Squibb, the Ichiro Kanehara Foundation, the Takeda Science Foundation, and the Takeda Foundation.
“In conclusion, p53 protects against formation of extrahepatic biliary precancerous lesions in the context of oncogenic Kras in mice, however, inactivation of p53 is not sufficient for the progression into invasive cancer in the extrahepatic biliary system.”
Click here to read the full editorial paper in Oncotarget.
—
Oncotarget is an open-access, peer-reviewed journal that has published primarily oncology-focused research papers since 2010. These papers are available to readers (at no cost and free of subscription barriers) in a continuous publishing format at Oncotarget.com. Oncotarget is indexed/archived on MEDLINE / PMC / PubMed.
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact media@impactjournals.com.