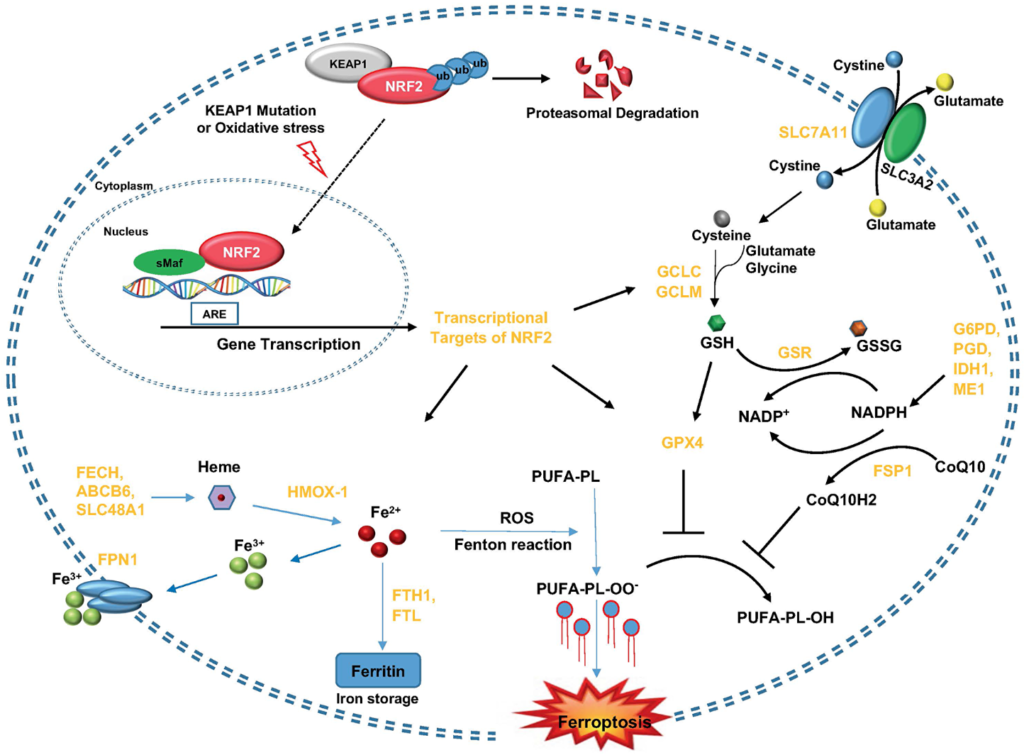
“In non–small cell lung cancer (NSCLC), alterations in the HER2 (ERBB2) gene define a unique molecular subtype.”
Lung cancer remains one of the leading causes of cancer-related deaths worldwide. Although precision medicine has improved outcomes for many patients, certain rare genetic mutations are still poorly understood, particularly in regions with limited access to genomic testing. Such mutations involve the HER2 gene, better known for its role in breast cancer but also implicated in a small subset of lung cancers.
HER2 mutations are found in approximately 2–4% of non-small cell lung cancer (NSCLC) cases and create unique challenges. These tumors can vary significantly in how they appear under a microscope and in how they respond to treatment. Adding to the complexity, most diagnostic and treatment guidelines are based on research from high-income countries, which may not reflect the genetic diversity seen in other parts of the world.
To help close this knowledge gap, researchers in Northeastern Brazil conducted one of the first detailed investigations into HER2-mutated NSCLC in Latin America. Their study, recently published in Volume 16 of Oncotarget, reveals a complex and often overlooked form of the disease, highlighting the need for broader access to targeted therapies in underserved populations.
The Study: HER2-Mutated NSCLC in Northeastern Brazil
In the study titled “Molecular landscape of HER2-mutated non-small cell lung cancer in Northeastern Brazil: Clinical, histopathological, and genomic insights,” researchers led by first authors Cleto Dantas Nogueira from the Federal University of Ceará and Argos Pathology Laboratory and Samuel Frota from Argos Pathology Laboratory, along with corresponding author Fabio Tavora from the previously mentioned institutions and Messejana Heart and Lung Hospital, analyzed 13 cases of HER2-mutated NSCLC. They used clinical, pathological, and genomic data.
The Results: A Complex Clinical and Molecular Landscape
The analyzed patients ranged in age from 34 to 82 years. More than half were women. About half had never smoked.
The research team discovered different HER2-related mutations. Most tumors carried the well-known A775_G776insYVMA insertion in exon 20 of the HER2 gene. However, rarer mutations such as V842I and Q709L were also identified, indicating substantial genetic diversity. More than half of the patients had additional mutations in other key cancer-related genes, especially TP53, a gene associated with aggressive tumor behavior and resistance to treatment.
Interestingly, most tumors did not overexpress the HER2 protein, even though they carried HER2 mutations. Only one patient showed strong protein expression based on standard immunohistochemistry (IHC) testing. This finding suggests that relying only on protein-level tests may miss cases that could benefit from targeted treatment. Additionally, all tumors had a low tumor mutation burden (TMB), which has been linked to limited effectiveness of immunotherapies.
Treatment access emerged as a major concern. Only one patient received trastuzumab deruxtecan, a promising new drug specifically designed for HER2-mutated cancers. Most were treated with surgery, chemotherapy, immunotherapy, or a combination of these approaches. While a few patients lived for years after diagnosis, most experienced rapid disease progression, especially those diagnosed at more advanced stages.
The Breakthrough: Mutations in Underserved Populations
This study underscores the molecular diversity of HER2-mutated NSCLC and highlights the importance of using comprehensive genetic testing, not just protein-level tests, to detect targetable mutations. It also shows that patients in underserved regions can harbor complex cancers that need personalized treatment approaches.
The Impact: Making the Case for Genomic Equity in Lung Cancer
This research has the potential to reshape NSCLC diagnosis and treatment strategies in Brazil and other low- to middle-income countries. By confirming that HER2 mutations are present in regions where they are rarely investigated, it strengthens the case for expanding access to next-generation sequencing and innovative targeted therapies like trastuzumab deruxtecan.
Future Perspectives and Conclusion
Although the study’s sample size was small, its implications are important. HER2-mutated NSCLC is more genetically diverse than previously recognized, and this variability must be reflected in both diagnostic and treatment strategies. The authors advocate for the establishment of regional molecular tumor boards to guide personalized care and increase access to clinical trials.
As more data becomes available, the goal is to tailor therapies not just to specific mutations but also to the unique characteristics of local patient populations, marking a crucial step toward more equitable cancer care worldwide.
Click here to read the full research paper published by Oncotarget.
_______
Oncotarget is an open-access, peer-reviewed journal that has published primarily oncology-focused research papers since 2010. These papers are available to readers (at no cost and free of subscription barriers) in a continuous publishing format at Oncotarget.com.
Oncotarget is indexed and archived by PubMed/Medline, PubMed Central, Scopus, EMBASE, META (Chan Zuckerberg Initiative) (2018-2022), and Dimensions (Digital Science).
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact media@impactjournals.com.