In a new review, researchers from The Hebrew University of Jerusalem discuss the challenges associated with targeting Ras proteins and how protein engineering has emerged as a promising method to overcome these challenges.
Ras plays a crucial role in controlling various cellular processes by switching between active (Ras-GTP) and inactive (Ras-GDP) states with the help of specific molecules. In its active form, Ras interacts with multiple effector proteins, initiating downstream events. Humans have three Ras genes, resulting in four isoforms that have distinct expression patterns and unique functions in different tissues. Posttranslational modifications target Ras to the cell membrane, where it can form dimers and interact with effectors through common domains. Ras mutations, commonly found in pancreatic, colorectal and lung cancers, lock Ras in an active state, promoting continuous cell division and proliferation. Ras signaling disruption occurs through reduced catalytic activity, altered effector binding and decreased affinity for other regulatory proteins.
Although Ras has been considered difficult to target, recent advancements have identified potential binding pockets that can be addressed by small molecules, peptidomimetics and proteins. Inhibitors designed to covalently bind to the Ras G12C mutant have shown promise, leading to FDA-approved drugs for specific lung cancers. Additionally, protein-based inhibitors that target Ras and its interactions with effectors, regulatory proteins and guanine nucleotide exchange factors offer alternative strategies for therapeutic intervention. These developments have challenged the notion that Ras is “undruggable” and highlight the potential for effective treatments against various cancer types.
On July 1, 2023, researchers Atilio Tomazini and Julia M. Shifman from The Hebrew University of Jerusalem published a new review paper in Oncotarget, entitled, “Targeting Ras with protein engineering.” The authors provide an overview of the challenges associated with targeting Ras proteins with small molecules and discuss how protein engineering has emerged as a promising method to overcome these challenges.
“While the development of small-molecule Ras inhibitors has been reviewed elsewhere [40], we focus our review on protein-based Ras inhibitors, describing the methods for their engineering, various scaffolds used for inhibitor design, and prospects for delivery of the designed Ras inhibitors into the cellular cytoplasm, where Ras is located.”
Protein Engineering
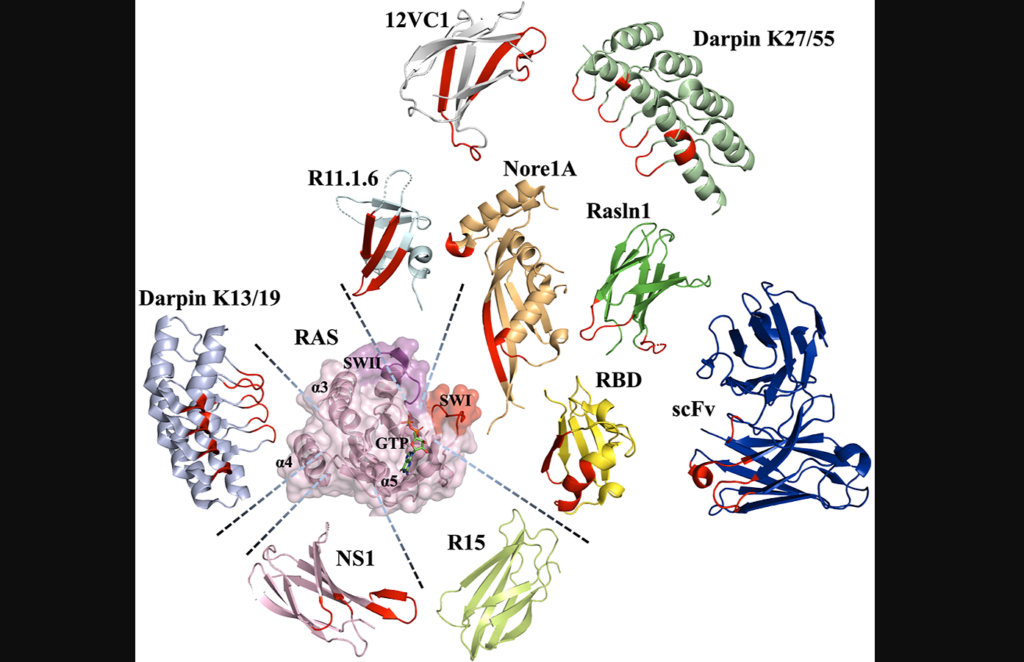
Protein scaffolds offer alternative approaches to small molecule drugs for engineering protein-based inhibitors. Unlike small molecules, protein domains can bind to targets through large surface areas, providing high affinity and specificity. Antibodies, natural protein effectors and novel binding domains are commonly used as protein scaffolds. Antibodies can be engineered into smaller versions to overcome limitations, while natural effectors can be modified to enhance binding affinity. Novel binding domains, unrelated to the target protein, possess structural robustness and can be evolved to exhibit strong binding. All three classes of protein scaffolds have been utilized to engineer Ras binders and explore strategies to inhibit Ras oncogenesis.
“Interestingly, all classes of protein scaffolds, including antibodies, natural effectors, and novel binding domains, have been utilized for engineering of Ras binders, allowing scientists to target various sites on the Ras surface and to explore different strategies for inhibiting Ras oncogenesis […].”
Methods for engineering protein inhibitors can be categorized into experimental directed evolution and computational design, or a combination of both. Experimental techniques involve display technologies such as phage display, yeast surface display, ribosome display, and mRNA display. These methods allow for the construction of combinatorial libraries of protein mutants, which are then screened using the target protein as a selection “bait.” The selected binders are sequenced to identify high-affinity mutants. Negative selection steps can be incorporated to enhance specificity by eliminating binders to unwanted targets. The number of mutants that can be assayed depends on the display technology used, with each approach having its limitations.
In addition to experimental approaches, computational methods have been proposed for protein binder design. Computational design enables rational targeting of specific binding epitopes on the target protein. However, computationally designed binders often have weak initial binding affinities and require affinity maturation through experimental techniques. Computational methods have been successful in designing focused libraries for yeast surface display experiments, where small libraries of protein mutants are designed based on computational predictions. This approach narrows down the choices to the most promising mutants, facilitating directed evolution experiments. By combining computational and experimental approaches, protein inhibitors with superior affinity and specificity have been developed.
“We have summarized all the described engineered Ras protein-based binders and their properties in Table 1.”
The Future of Intracellular Transport for Ras Inhibitors
Efficient delivery of molecules that bind to intracellular Ras proteins is essential for suppressing pro-cancer pathways and promoting anti-cancer activities. To overcome the challenge of crossing the cell membrane, different strategies have emerged. One approach involves utilizing short cell-penetrating peptides (CPPs) that can be fused to the desired protein, allowing entry into cells through direct translocation or endocytosis. However, improving the release of cargo proteins from endosomes remains a hurdle. Supercharging proteins with positively charged surfaces or leveraging bacterial toxins with intrinsic delivery mechanisms are alternative methods for intracellular protein delivery. Additionally, coupling cargo proteins to nanoparticles or employing mRNA delivery systems have shown promise, although they have their own limitations.
These protein delivery techniques have been explored for targeting Ras inhibitors. For instance, a human IgG1 antibody was engineered to selectively bind to Ras-GTP, inhibiting downstream signaling. Fusion of Ras binding domains to CPPs demonstrated competitive inhibition of Ras/effector interactions. Furthermore, optimized bacterial secretion systems and lipid nanoparticle-encapsulated mRNA platforms have been employed for efficient intracellular delivery of Ras-binding molecules. These advancements open up possibilities for targeted cancer therapies and disease treatments by enabling effective delivery of Ras binders to their intracellular target, thus influencing cancer-related signaling pathways.
Conclusions
In summary, targeting Ras proteins, despite their historically challenging nature, has seen significant progress in recent years. Small molecules, peptidomimetics and protein-based inhibitors have emerged as potential strategies for inhibiting Ras oncogenesis. Protein engineering, utilizing various protein scaffolds such as antibodies, natural effectors and novel binding domains, offers alternative approaches to traditional small molecule drugs.
Experimental directed evolution and computational design, alone or in combination, have facilitated the development of high-affinity and specific protein inhibitors. Furthermore, the efficient intracellular delivery methods described above hold promise for targeted cancer therapies by effectively delivering Ras binders to their intracellular targets. These advancements challenge the perception of Ras as “undruggable” and provide hope for the development of effective treatments for various cancer types.
“These strategies should be utilized in future to examine the beneficial activity of Ras-binders and inhibitors and should further facilitate the development of protein-based Ras therapeutics.”
Click here to read the full review in Oncotarget.
ONCOTARGET VIDEOS: YouTube | LabTube | Oncotarget.com
—
Oncotarget is an open-access, peer-reviewed journal that has published primarily oncology-focused research papers since 2010. These papers are available to readers (at no cost and free of subscription barriers) in a continuous publishing format at Oncotarget.com. Oncotarget is indexed/archived on MEDLINE / PMC / PubMed.
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact media@impactjournals.com.
