In a new editorial, researchers discuss opening the blood-brain barrier and a promising new strategy for the treatment of brain cancer.
Just a small number of molecules, including alcohol and caffeine, are able to cross the blood-brain barrier (BBB). The BBB is a highly selective semipermeable membrane that separates circulating blood from extracellular fluid in the brain. It plays a critical role in protecting the brain from harmful substances in the blood while also maintaining a stable and consistent environment for neuronal function. Without the BBB, humans would be at the mercy of any harmful toxin, pathogen and unwanted substance that could cross from the bloodstream into the brain.
This protective function also makes it difficult to deliver therapeutic agents to the brain, as the majority of drugs and other molecules are unable to cross the BBB. This is particularly problematic for the treatment of brain-localized diseases, including brain cancers and neurological disorders, which require high concentrations of drugs to effectively target sites in the brain. In a new editorial paper, researchers Thomas C. Chen, Weijun Wang and Axel H. Schönthal from the University of Southern California‘s Keck School of Medicine discuss a series of preclinical studies that introduced the novel concept of intraarterial (IA) injection of NEO100—a promising strategy aimed at temporarily and safely opening the BBB up for therapeutic treatment. Their editorial was published in Oncotarget’s Volume 14 on May 4, 2023, entitled, “From the groin to the brain: a transfemoral path to blood-brain barrier opening.”
“It is believed that procedures to open the BBB in a controlled and safe fashion might provide tremendous advantages by allowing optimal brain entry of any and all circulating therapeutics.”
Opening the BBB
The authors first describe previously used methods of opening the BBB for therapeutic intervention, including intracarotid injection of hyperosmolar mannitol and MRI-guided pFUS with intravascular microbubbles. Unfortunately, these methods have yielded issues with safety and efficacy. Fortunately, Chen, Wang, Schönthal, and their co-authors came up with a new idea for opening the BBB safely.
In a 2021 study, the researchers discovered that NEO100 enables the delivery of BBB-impermeable therapeutics to the brain. NEO100 is a type of perillyl alcohol—a natural chemical found in citrus fruit peels—that has been studied for its potential to treat cancer. Wang et al. aimed to see if injecting NEO100 into an artery would open the BBB safely and temporarily. This could help other drugs that are normally unable to pass through the BBB, such as methotrexate and therapeutic antibodies, to enter the brain. Previously, NEO100 had been administered through the nose to treat cancer, but this study focused on its ability to open the BBB.
The researchers injected NEO100 into the left ventricle of the heart and then injected a dye called Evans blue into the mice’s veins. Normally, this dye cannot penetrate the brain, but when the BBB is weakened or opened up, it can get through and turn the brain blue. And that’s exactly what happened—the mice’s brains turned blue after the injections. Interestingly, when they tried using another substance called mannitol, it did not have the same effect on the BBB. The team performed additional studies and found that NEO100 seemed to affect the connections between cells in the barrier.
In further experiments, the researchers used methotrexate and special markers that usually do not enter the brain. They gave these drugs and markers to mice and found that NEO100 made it easier for the drugs and markers to enter the brain. This effect lasted between two and four hours before the BBB reverted to normal functioning. The researchers also tested administering NEO100 by injecting it into the mouse’s veins, but this was not effective.
The main question the researchers wanted to answer was if opening the BBB using IA NEO100 could help treat brain tumors. To answer this question, they conducted experiments using mice that had tumor cells implanted in their brains. In one study, they used breast cancer cells that were engineered to have the protein HER2 and treated them with trastuzumab. In another study, they used models of brain cancer called melanoma and glioblastoma and treated them with drugs that help the immune system fight cancer. These studies have found a way to improve drug delivery for CNS diseases, but there are limitations that need further investigation.
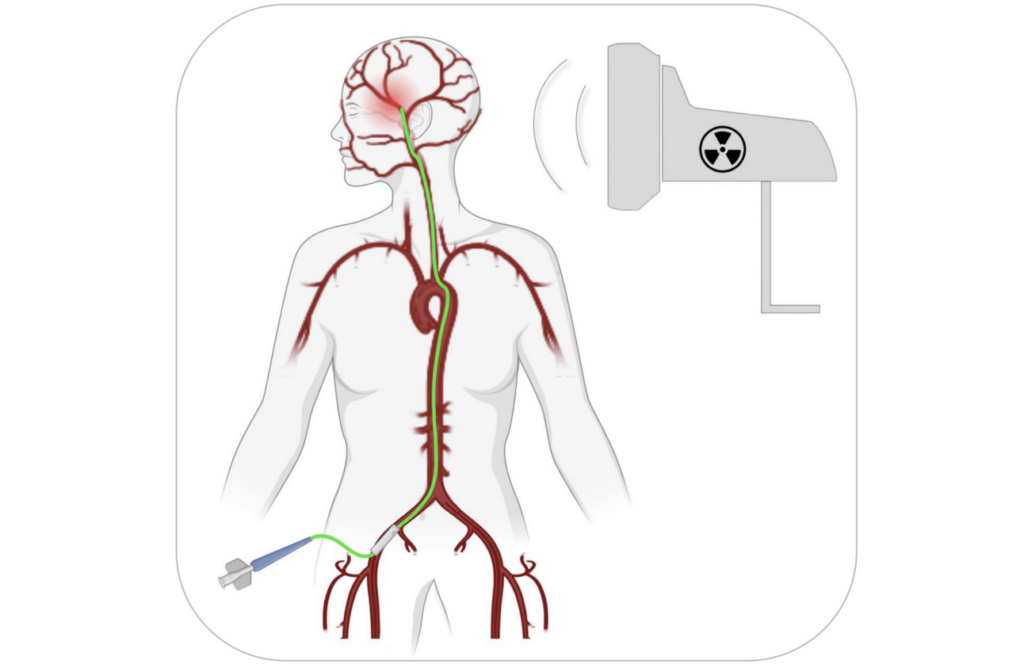
Transfemoral IA catheterization
As noted in this editorial, the preclinical models above used one injection of NEO100 with a therapeutic agent, but it’s unclear if this will work as well in humans. Tumors in humans are more complex than in rodents, so multiple interventions might be needed. It is also important to determine the best way to perform the injection(s) in humans. The researchers suggest using a catheter inserted through the femoral artery near the groin and guided by fluoroscopy to safely inject NEO100 into the cranial arteries.
“Transfemoral IA catherization (Figure 1) is a low-risk procedure that is routinely performed by endovascular neurosurgeons in the context of cerebral angiograms, aneurysm coiling, tumor embolization, and thrombectomies [18]. It is considered ‘the gold standard technique for catheter-based neuro-interventions’ [19]. However, it has never been used as a means to access tumor-feeding cranial arteries for purposes of BBB opening.”
Transfemoral IA catheterization is a medical procedure that involves inserting a catheter through a blood vessel in the leg and guiding it to the brain to perform various treatments. It is a safe and common technique, already used by doctors who specialize in treating brain conditions. However, it has never been used to open the BBB in order to access the blood vessels. Using NEO100 with this procedure could be a new and innovative way to treat aggressive brain tumors. If necessary, the procedure could even be repeated multiple times due to its safe and simplistic nature. The researchers believe that using this new method to open the BBB could be just as successful in treating brain tumors as current treatments are for tumors in other parts of the body. This could potentially lead to better outcomes for patients with brain tumors, such as improved survival rates and fewer side effects.
Conclusions
The blood-brain barrier (BBB) is a protective barrier that prevents harmful substances from entering the brain. However, this barrier also makes it difficult to deliver therapeutic agents to the brain. In a new study, researchers have proposed a novel method of intraarterial injection of NEO100 to temporarily and safely open the BBB. This method has been shown to enable the delivery of BBB-impermeable therapeutics to the brain. The authors of this editorial have suggested using transfemoral IA catheterization to perform this intervention. The method requires further investigation and development.
“The authors envision that clinical implementation of this new BBB-opening method might achieve a similarly high rate of success in the treatment of brain-localized malignancies as do current treatments for peripherally distributed tumors; as a result, reduced morbidity and increased patient survival is expected.”
Click here to read the full editorial in Oncotarget.
ONCOTARGET VIDEOS: YouTube | LabTube | Oncotarget.com
—
Oncotarget is an open-access, peer-reviewed journal that has published primarily oncology-focused research papers since 2010. These papers are available to readers (at no cost and free of subscription barriers) in a continuous publishing format at Oncotarget.com. Oncotarget is indexed/archived on MEDLINE / PMC / PubMed.
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact media@impactjournals.com.