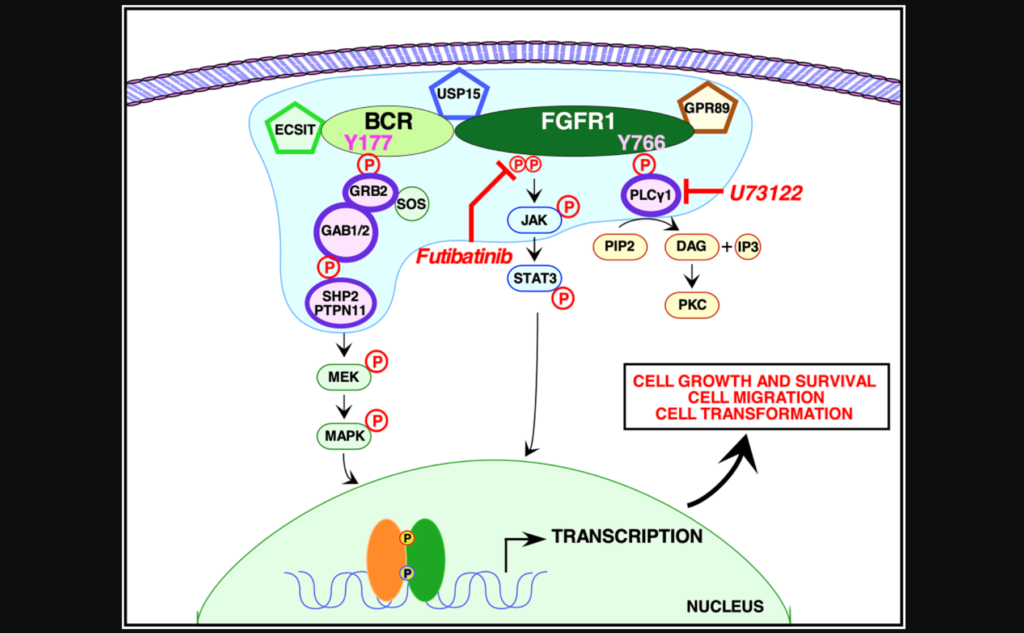
For the first time, researchers revealed the protein interactome, phospho-proteome and total proteome for the oncogenic fusion protein BCR-FGFR1.
The Trending With Impact series highlights Oncotarget publications attracting higher visibility among readers around the world online, in the news, and on social media—beyond normal readership levels. Look for future science news about the latest trending publications here, and at Oncotarget.com.
—
Chromosomes are found in the nucleus of cells and consist of proteins and tightly coiled strands of DNA. During cell division, chromosomal translocations can occur while the chromosomes are being copied. This type of mutation can mean that an entire chromosome has moved to another location, or that a chromosome has broken, usually into two pieces, and moved to another site. Some translocations are harmless, but others can lead to aberrant cell proliferation and cancer.
“Over the last half century, chromosomal translocations encoding functional oncogenic proteins have been identified as drivers of multiple cancers, and account for 20% of all malignant neoplasms [1, 2].”
For example, the t(8;22)(p11;q11) chromosomal translocation leads to the initiation of an oncogenic fusion protein called the Breakpoint Cluster Region Fibroblast Growth Factor Receptor 1 (BCR-FGFR1). BCR-FGFR1 is a single driver of 8p11 myeloproliferative syndrome, which is also known as stem cell leukemia/lymphoma (SCLL).
“Stem cell leukemia/lymphoma (SCLL) exhibits distinct clinical and pathological features characterized by chromosomal translocations involving the FGFR1 gene at chromosome 8p11.”
In a trending new study, researchers from the University of California San Diego and Sanford Burnham Prebys Medical Discovery Institute examined mutations in PLCγ1 and Grb2 binding sites individually and when combined together in a double mutant within BCR-FGFR1. On May 11, 2022, the research paper was published in Oncotarget and entitled, “Proteomic analysis reveals dual requirement for Grb2 and PLCγ1 interactions for BCR-FGFR1-Driven 8p11 cell proliferation.”
The Study
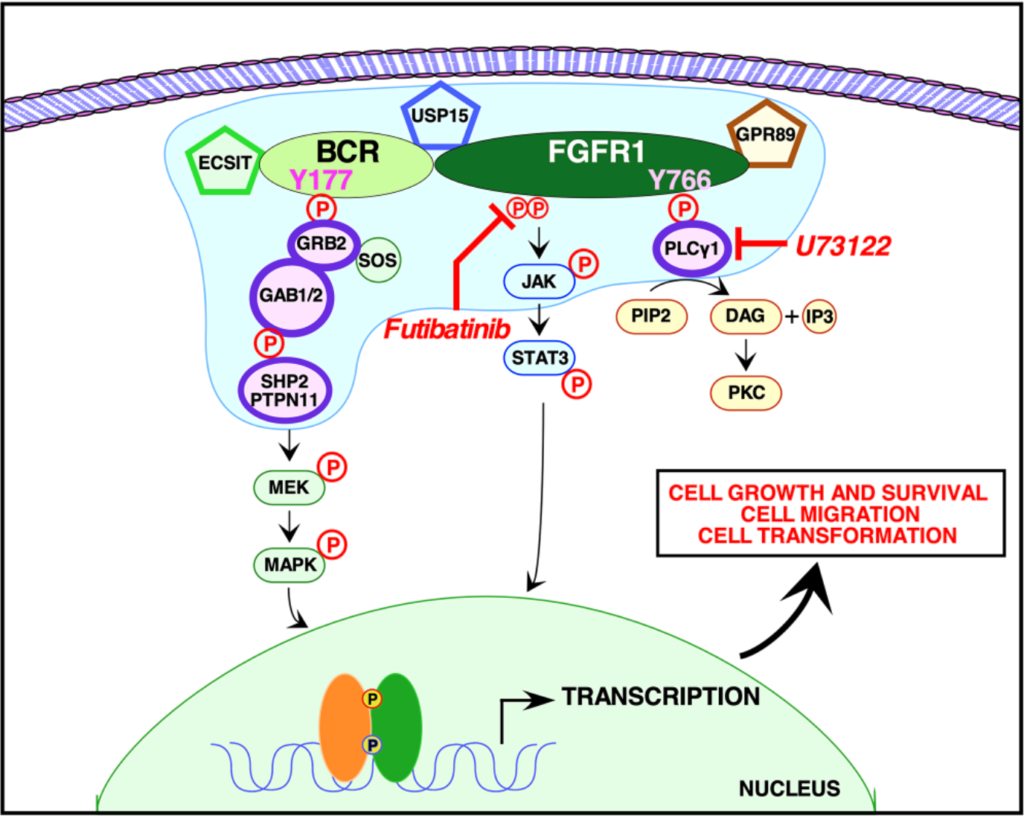
In this study, the researchers used quantitative proteomic analyses to identify the crucial protein-to-protein interactions that may be necessary to activate BCR-FGFR1. The team used NIH3T3, HEK293T and 32D cells to assay five types of mutations: wild type BCR-FGFR1, a kinase-dead variant of BCR-FGFR1, a derivative of BCR-FGFR1 that contained a single mutation abolishing the Grb2 interaction site, a derivative of BCR-FGFR1 that contained a single mutation abolishing the PLCγ1 interaction site, and a double mutation that abolished both interaction sites (BCR(Y177F)-FGFR1(Y766F)).
“These data demonstrate that inhibition of either signaling pathway alone fails to inhibit hematopoietic cell proliferation, and demonstrate a dual requirement for Grb2 and PLCγ1 interactions with BCR-FGFR1 for proliferation.”
When either Grb2 or PLCγ1 signaling pathway was mutated, BCR-FGFR1 activity was decreased, but never abolished. However, when both Grb2 and PLCγ1 interactions were mutated, both cell transformation and proliferation were inhibited. The team demonstrated that BCR-FGFR1 dually relies on Grb2 and PLCγ1 for biological activity and the activation of cell signaling pathways. The researchers also found that the PLCγ1 inhibitor U73122 revealed that PLCγ1 is a potential therapeutic target for BCR-FGFR1-driven hematologic malignancies. In addition, the irreversible FGFR inhibitor futibatinib suppressed downstream signaling and cell transformation.
“We demonstrate here that BCR-FGFR1 relies dually on the small adapter protein, Grb2, and the phospholipase, PLCγ1, for biological activity and the activation of cell signaling pathways (summarized in Figure 6).”
Conclusion
“Our work highlights the importance of sequencing based, mutation-specific therapies for FGFR1 induced hematologic malignancies.”
This study provides new insight into the potential molecular mechanisms underlying BCR-FGFR1 activity and identifies PLCγ1 as a therapeutic target for leukemia/lymphoma patients with this particular mutation. Future studies will be necessary to validate these findings in animal models and clinical trials. However, this study lays the groundwork for the development of new and more targeted leukemia/lymphoma therapies.
“These data unravel essential roles of Grb2 and PLCγ1 in BCR-FGFR1 mediated oncogenic growth and suggest the importance of further investigation into PLCγ1 as a potential therapeutic target in treating SCLL.”
Click here to read the full research paper published by Oncotarget.
ONCOTARGET VIDEOS: YouTube | LabTube | Oncotarget.com
—
Oncotarget is an open-access journal that publishes primarily oncology-focused research papers in a continuous publishing format. These papers are available at no cost to readers on Oncotarget.com. Open-access journals have the power to benefit humanity from the inside out by rapidly disseminating information that may be freely shared with researchers, colleagues, family, and friends around the world.
For media inquiries, please contact media@impactjournals.com.