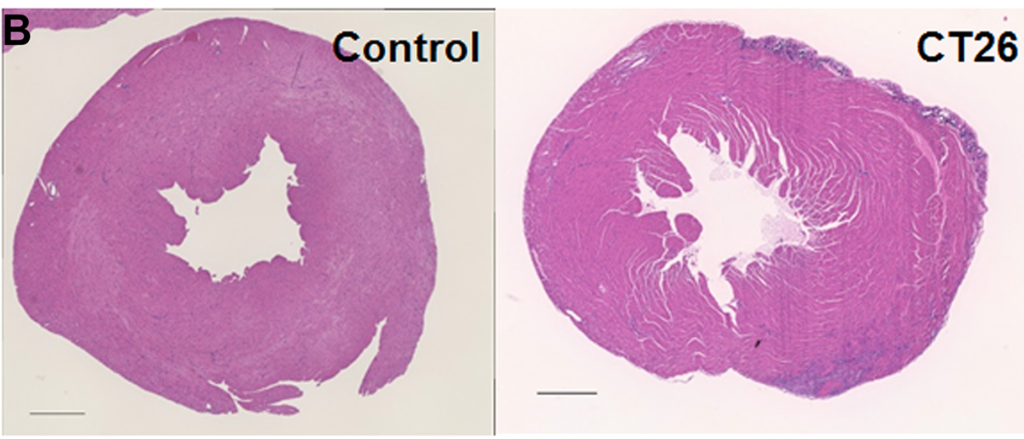
Researchers in this study employed one of the few available murine cachexia models and validated its ability to be used in future studies of cancer-derived myocardial damage.
The Trending with Impact series highlights Oncotarget publications attracting higher visibility among readers around the world online, in the news, and on social media—beyond normal readership levels. Look for future science news and articles about the latest trending publications here, and at Oncotarget.com.
—
Cachexia, a complex metabolic syndrome characterized in part by the loss of muscle mass, can account for up to 30% of all cancer-related deaths. Myocardial atrophy, or cardiac remodeling/degradation, is a phenotype of cachexia and a common cause of death.
“The causes of cancer-derived myocardial impairment might be the effects of cancer itself, background heart disease, and influence of cancer treatments; however, they have not been given much clinical importance, and specific treatment efforts are delayed [8].”
Researchers from Nara Medical University, Hanna Central Hospital, and Hoshida Minami Hospital in Nara and Osaka, Japan, and Nantong University in Jiangsu Province, China, note that while myocardial damage in cancer patients is known to be a cause of death, there are few murine cachexia models available to evaluate cancer-derived heart disorders. Thus, there is a need for further studies that may allow researchers to establish an intervention to prevent myocardial damage in cancer patients.
“In this study, we used the mouse cancer cachexia model that we previously established [14] to examine the status of cancer-derived myocardial impairment reported in literature, and validate our model for studying cancer-derived myocardial impairment.”
The Study
Some causes of cancer-derived myocardial impairment have been reported as cancer-induced cytokines, oxidative stress, depletion of antioxidants, and protein catabolism as a result of AKT/mTOR inhibition.
“Despite these advances in our understanding, the multifactorial mechanisms underlying cancer-derived myocardial impairment remain incompletely understood, necessitating further investigations to elucidate the molecular mechanisms and prevent myocardial damage in cancer patients.”
The researchers previously established a mouse cancer cachexia model. In this study, they aimed to validate their model by employing it in the examination of cancer-derived myocardial impairment that has been reported in previous literature. Their study enlisted the mouse model, CT26 colon cancer cell cultures, protein extraction, histological analysis, immunoblot analysis, enzyme-linked immunosorbent assay (ELISA), mitochondrial stress tests (Seahorse assay), glycolytic stress tests, and statistical analysis.
Conclusion
“In summary, our established mouse cachexia model showed various myocardial changes associated with cancer cachexia such as oxidative stress in the myocardium, energy metabolism, autophagy, and inflammatory cytokines.”
Results obtained by the researchers in this study using their mouse cachexia model are congruent with previously reported results about cancerous myocardial damage, and therefore provide reasonable evidence that it may be used in future studies.
“The established mouse cachexia model can therefore be considered useful for analyzing cancer-derived myocardial damage.”
Click here to read the full scientific study, published in Oncotarget.
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read—without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared with friends, neighbors, colleagues and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.