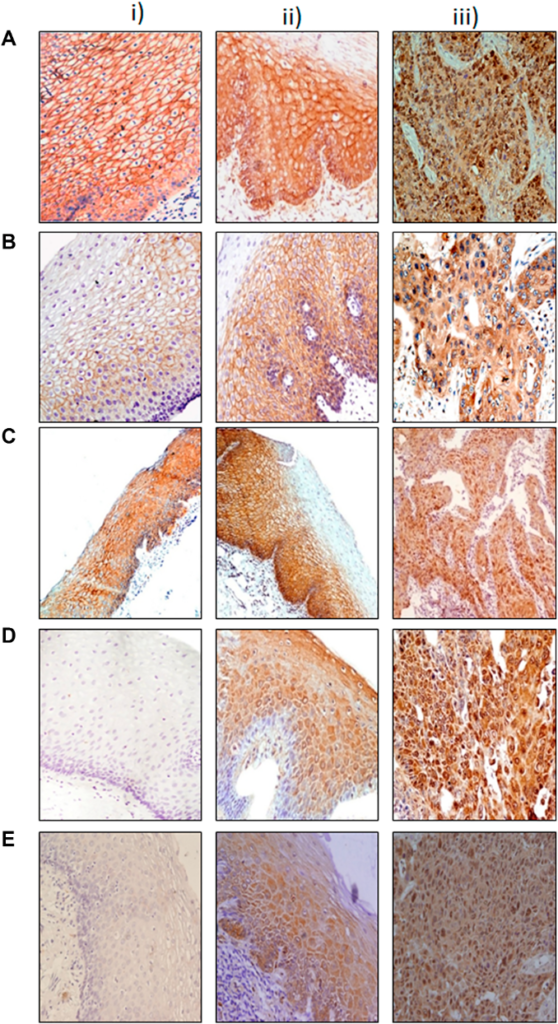
In a new study, researchers evaluated the high altitude in Ecuador and how it may influence HIF-1 expression and the survival of Ecuadorian patients with gastric cancer.

Altitude is considered any elevation above sea level. Higher altitude environments are known to influence various physiological processes in the human body, including those related to hypoxia-inducing factors (HIF), vitamin D, ultraviolet radiation, oxygen toxicity, and changes in pH. Researchers have suggested that altitude may even affect the development and progression of some diseases, including stomach/gastric cancer.
“Gastric cancer is the third leading cause of death in the world and is estimated to cause almost 15 million deaths by 2035 [2].”
Gastric Cancer & Altitude
The primary subtype of gastric cancer is gastric adenocarcinoma (GA). GA develops in the mucus-secreting cells that line the stomach (gastric epithelium). Higher incidence rates of GA have been found among populations living at high altitudes. High altitudes are notorious for low air pressure and decreased oxygen saturation levels. Decreases in oxygen (hypoxia) can activate the transcriptional regulator hypoxia-inducing factor-1 (HIF-1). HIF-1 is known to be upregulated in a variety of human cancers, including GA. The role of HIF-1 in GA pathogenesis and prognosis has not yet been fully understood.
“Gastric adenocarcinoma (GA) has a high incidence in Ecuador, in men it ranks third and in women it ranks fifth.”
There is a higher incidence of GA among people living in Ecuador. This is a country that straddles the equator yet, the altitude in Ecuador varies significantly across the country. For example, the altitude is 2,850 meters in the capital city of Quito (the second-highest capital city in the world). In Guayaquil (a coastal city in Ecuador) the altitude is only nine meters. These facts make Ecuador an optimal location for studying the effects of altitude on gastric adenocarcinoma.
“Ecuador has a varied altitude diversity and there is a differential incidence of cancer between populations living in the Andean or mountainous region when compared to coastal populations or living at low altitude.”
The Study
In a new retrospective study, researchers Edwin Cevallos Barrera, Edson Zangiacomi Martinez, Mariangela Ottoboni Brunaldi, Eduardo Antonio Donadi, Ajith Kumar Sankarankutty, Rafael Kemp, and José Sebastiao dos Santos from Universidad Central del Ecuador and University of São Paulo evaluated the high altitude in Ecuador and how it may influence HIF-1 expression and the survival of Ecuadorian patients with GA. Their research paper was published on September 14, 2022, in Oncotarget’s Volume 13, entitled, “Influence of high altitude on the expression of HIF-1 and on the prognosis of Ecuadorian patients with gastric adenocarcinoma.”
A total of 229 Ecuadorians were assessed in this study. The researchers included 155 cases of GA; 99 of the individuals with GA lived in the mountainous regions of Quito and Ambato, and 56 individuals lived in the coastal region of Guayaquil. (Controls accounted for 74 people; 25 from the coast and 49 from the mountains.) The team followed-up with all individuals in this study from 2005 to 2018 and collected blood and tissue samples. They performed immunohistochemistry and other analyses to evaluate HER2 and HIF-1 expression.
“Analyses were performed using Fisher’s exact and Breslow-Day tests for homogeneity and Kaplan-Meier curves and restricted median survival time ΔRMST.”
Results & Conclusion
After 10 years, median survival was significantly higher among GA patients living along the coast. In the GA samples, HIF-1 was observed in 66.1% of the coastal patients and in 43.4% of the mountainous patients. Positive HIF-1 expression was associated with improved survival among GA patients living in the mountains. Interestingly, in the control group (without GA), HIF-1 expression was observed in 95.9% of the mountainous samples and only 32% of the coastal samples. Their results showed that 89% of the control population exhibited gastritis.
The researchers were forthcoming about the limitations of this study. Differences in quantities and the male-to-female ratios within the GA and control coastal and mountainous groups may have influenced data. Despite these limitations, the researchers observed that coastal GA patients and individuals who expressed HIF-1 had a better prognosis. However, HIF-1 expression was only associated with better survival in the mountain region. These findings suggest that HIF-1 expression may be a protective factor against GA progression in people living at high altitudes.
“Concluding, this study suggests that HIF-1 has a differential expression pattern in gastric samples according to geographical features, being highly expressed even in non-carcinomatous cells (gastritis and normal mucosa) from individuals living in regions of high altitude, indicating that the gastric HIF-1 expression may be an adaptation of the individual to high altitudes.”
Click here to read the full research paper published by Oncotarget.
ONCOTARGET VIDEOS: YouTube | LabTube | Oncotarget.com
—
Oncotarget is an open-access journal that publishes primarily oncology-focused research papers in a continuous publishing format. These papers are available at no cost to readers on Oncotarget.com. Open-access journals have the power to benefit humanity from the inside out by rapidly disseminating information that may be freely shared with researchers, colleagues, family, and friends around the world.
For media inquiries, please contact media@impactjournals.com.